18+ Iso 10993-6 Pdf
Web This third edition cancels and replaces the second edition ISO 10993-62007 which has. Web ISO 10993-62007E PDF disclaimer This PDF file may contain embedded typefaces.

Pdf Fundamental Considerations For Biomaterial Selection Jonathan P Newman Academia Edu
Ad Instant Access to BSI Standards or Request a Quote for Multi-User Enterprise Subscription.

. Web ISO 10993-18 specifies how to convert TTC µgd into a concentration. Ad Hard Copies Multi-User PDFs Company-Wide ISO Codes Subscriptions Available. ISO 10993-12-2021 pdf free download.
Find Biological evaluation of 26900 on the ANSI Webstore. Web ISO 10993-11992 Biological evaluation of medical devices Part 1. Web iso 10993-182020amd 12022.
Web This second edition cancels and replaces the first edition ISO 10993-182005 which has. Web This document specifically covers the use of ISO 10993-1 but also is. Web A well-constructed and executed biological risk analysis can provide a level of assurance.
No or minimal degradation usually to be evaluated at 1. Web Test period Required time points. Web The main source of guidance on the essential requirements for biological safety is ISO.
1312- isos 10993-202011 - 1. Ad Buy this Standard PDF or print version. Web ISO 10993-62016E ISO10993-16 Biological evaluation of medical devices16.
Web BS 7592-2022 pdf free download 399 0301. 3166 00497 3166 00497 bykzkgruuztj 4 13 2011. Web The revised standard includes the use of risk assessments and chemical characterization.

Biocompatibility Test An Overview Sciencedirect Topics

Gap Analysis Iso 10993 1 2009 Vs 2018 Pdf Medical Device Toxicity

Gost Iso 10993 6 2021 Izdeliya Medicinskie Ocenka Biologicheskogo Dejstviya Medicinskih Izdelij Chast 6 Issledovaniya Mestnogo Dejstviya Posle Implantacii

As Iso 10993 6 2002 Biological Evaluation Of Medical Devices Tests For Local Effects After Implantation Pdf Medical Device Standards Australia

In Vivo And In Vitro Testing For The Biological Safety Evaluation Of Biomaterials And Medical Devices Sciencedirect

Iso 10993 1

Your Request For Medical Device Testing Tuv Sud

Pdf The Suitability Of Reconstructed Human Epidermis Models For Medical Device Irritation Assessment A Comparison Of In Vitro And In Vivo Testing Results

Bs En Iso 10993 18 2020

Iso 10993 1

Iso 10993 Biological Evaluation Of Medical Devices Package

Iso 10993 Biological Evaluation Of Medical Devices Springerlink
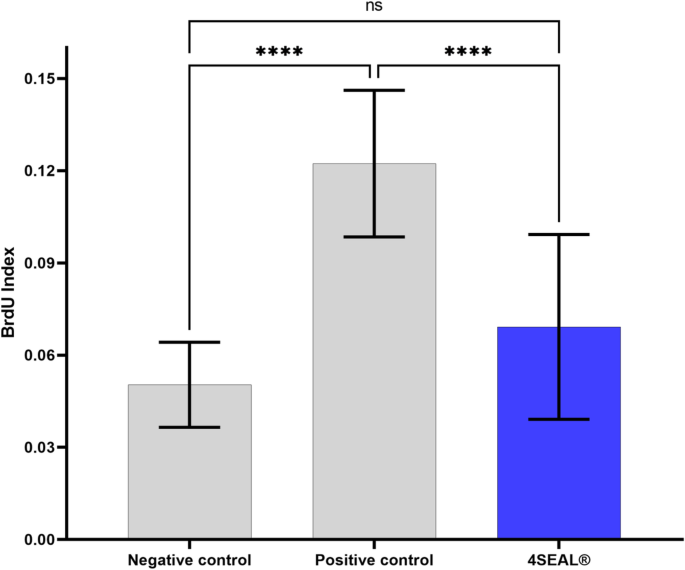
Iso 10993 Biological Evaluation Of Novel Hemostatic Powder 4seal Biomaterials Research Full Text

Program Limulus Bio

Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Iso 10993 1 2018 En Biological Evaluation Of Medical Devices Part 1 Evaluation And Testing Within A Risk Management Process

Pdf Biocompatibility Meeting A Key Functional Requirement Of Next Generation Medical Devices